India: the Land of Obesity

India has witnessed a significant increase in the rates of obesity in recent years. According to the National Family Health Survey-5 (NFHS-5), one in every four Indians is now overweight or obese (Figure 1) (Kalra et al., 2023). Projections indicate that by 2030, almost 64 million Indians will live with obesity, which will place India third in the world after the U.S. and China (Singh et.al.,2023).
Obesity and excess weight significantly raise the risk of chronic illnesses like diabetes, heart disease, high blood pressure, and certain cancers—the leading causes of death worldwide. In 2025, health authorities revised the definition of obesity for Asian Indians to reflect the higher risk of cardiometabolic diseases at lower BMI in South Asians. In response to this rising burden, the role of lifestyle interventions and the introduction of new weight loss drugs in India are gaining increasing attention as potential solutions.
This blog provides a concise and insightful overview of how new weight loss drugs in India are transforming obesity care in the country.
GLP-1 RAs – in Obesity Management
The Introduction Of New Incretin-Based Therapies has transformed Current Advancements In The Management Of Obesity. The intestine produces natural hormones called incretins that help control blood sugar levels and appetite. The two main types of incretin hormones are Glucagon-Like Peptide-1(GLP-1) and Glucose-Dependent Insulinotropic Polypeptide (GIP). To Capitalise on these benefits in individuals with type 2 diabetes and obesity, scientists have created a class of medicines called GLP-1 Receptor agonists (GLP-1 RAs), which mimic the action of the natural GLP-1 hormone. (Jang et al.,2024). The first GLP-1 RA drug for weight management, liraglutide, was approved by the U.S. FDA in 2014 under the brand name Saxenda, with once-daily administration. Researchers made minor structural modifications to liraglutide to develop semaglutide, resulting in greater effectiveness and enabling once-weekly dosing. (Jenstrele et.al.,2002).“Since the introduction of semaglutide, the landscape of weight management has transformed significantly.”
Semaglutide and Tirzepatide – Dawn of a New Era
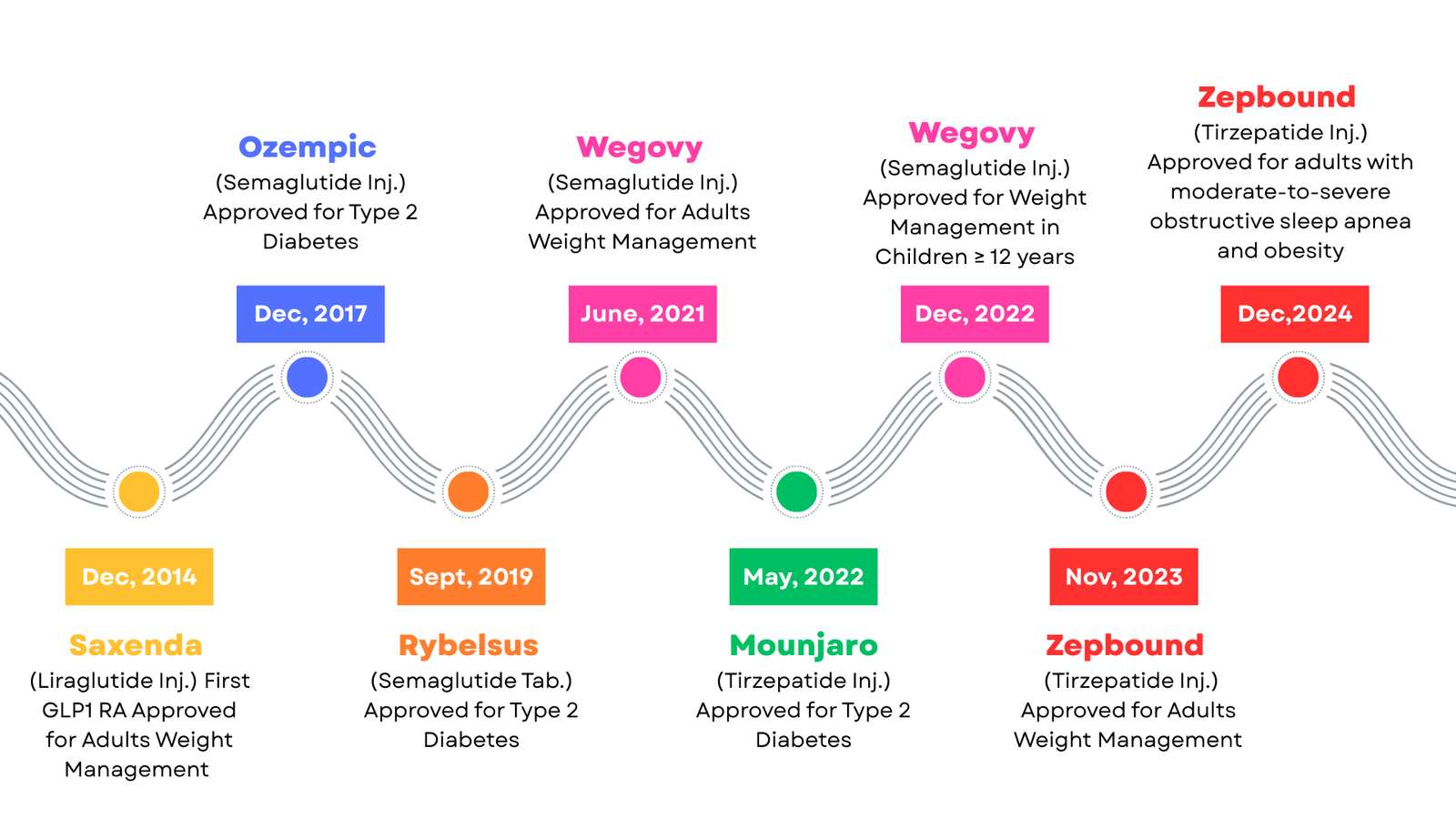
Semaglutide, administered via subcutaneous injection, received FDA approval in December 2017 under the brand name Ozempic for the treatment of type 2 diabetes. Since then, the FDA has expanded its use for chronic obesity, enabling healthcare providers to prescribe it for weight loss. In September 2019, the FDA approved the oral formulation of semaglutide, branded as Rybelsus, to help individuals with type 2 diabetes manage their blood glucose levels. Novo Nordisk achieved another milestone in June 2021, when the FDA approved Wegovy for the treatment of obesity in adults. The agency further extended this approval in December 2022, authorizing Wegovy for adolescents aged 12 years and older who meet BMI criteria (Figure 2) (Wojtara et.al.,2023)
Tirzepatide is available as a subcutaneous injection under the brand names Mounjaro and zepbound. Eli Lilly earned FDA approval for Mounjaro in May 2022 to treat type 2 diabetes, making it the first medication to activate both the GLP‑1 and glucose-dependent insulinotropic polypeptide (GIP) receptors simultaneously—hence also known as a “Twincretin”(Wojtara et.al.,2023). In November 2023, the FDA approved Zepbound injection for chronic weight management in adults with obesity, based on clinical evidence demonstrating significant weight loss outcomes (FDA News Release). Then, in December 2024, the FDA approved Zepbound for moderate-to-severe obstructive sleep apnea in adults with obesity, making it the first prescription medication authorized to treat this condition (Figure 2) (Reuters, December,2024 ).
Eli Lilly launched Mounjaro in India in March 2025. Subsequently, three months later, in June 2025, Novo Nordisk unveiled its blockbuster weight-loss drug, Wegovy, in India. It is important to note that Ozempic is not officially available in the Indian market.
Semaglutide and Tirzepatide – Market Insights
Global Market Trends
According to a 2025 GlobeNewswire report, the global anti-obesity drug market is expected to grow from $12.8 billion in 2024 to $104.9 billion by 2035. Semaglutide and Tirzepatide remain the key drugs propelling this growth. North America currently holds about 60% of the total market share, making it the leading regional contributor. Semaglutide remains the leading player in the global GLP-1 obesity drug market, accounting for approximately 60.7% of total revenue in 2024, as reported by Grand View Research. Tirzepatide is also rapidly growing.
Indian Market Trends
The Indian obesity drug market has expanded fivefold since 2021, reaching a value of ₹ 6.28 billion. Lilly’s Mounjaro (tirzepatide) has gained an early advantage. Since its launch in late March, sales have increased, Doubling In June To Nearly 88,000 Units, Worth ₹260 million, compared to May. From March to May, Lilly sold approximately 81,500 units. In contrast, Novo Nordisk’s Wegovy entered the market in late June, with just 1,788 units sold, generating ₹25.3 million in sales (Reuters,July 2025).
GLP-1 RAs – Mechanism of Action
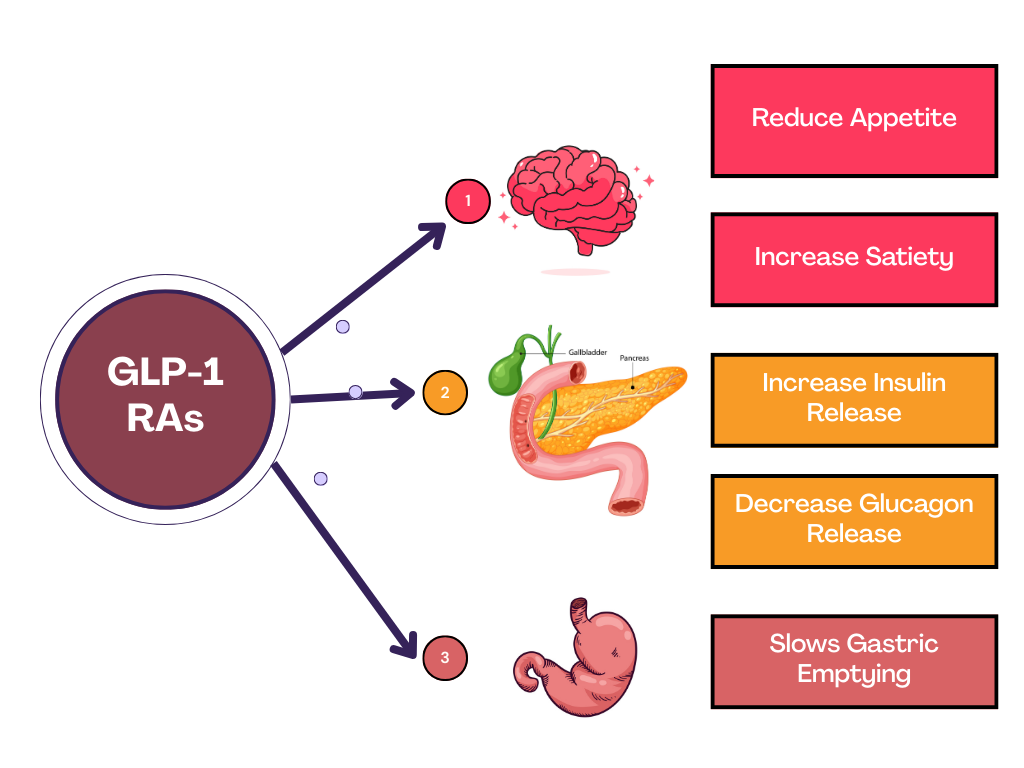
GLP-1 RAs Work by mimicking the Action of a Natural Hormone Called GLP-1, which is produced in the small intestine and plays a key role in controlling blood sugar levels and appetite (Figure 3). These medications help the pancreas release more insulin when blood sugar levels are high and reduce the release of glucagon, a hormone that raises blood sugar levels (Shankar et al., 2024). They also act on the brain to reduce Appetite or hunger and increase satiety—the feeling of fullness after eating. This helps people eat less and take in fewer calories. Additionally, GLP-1 RAs slow down gastric emptying, thus food leaves the Gut slowly. This prolongs the feeling of fullness and helps prevent sharp spikes in blood sugar after meals (Moiz, Areesha et.al.,2025).
Semaglutide vs Tirzepatide – Key Differences
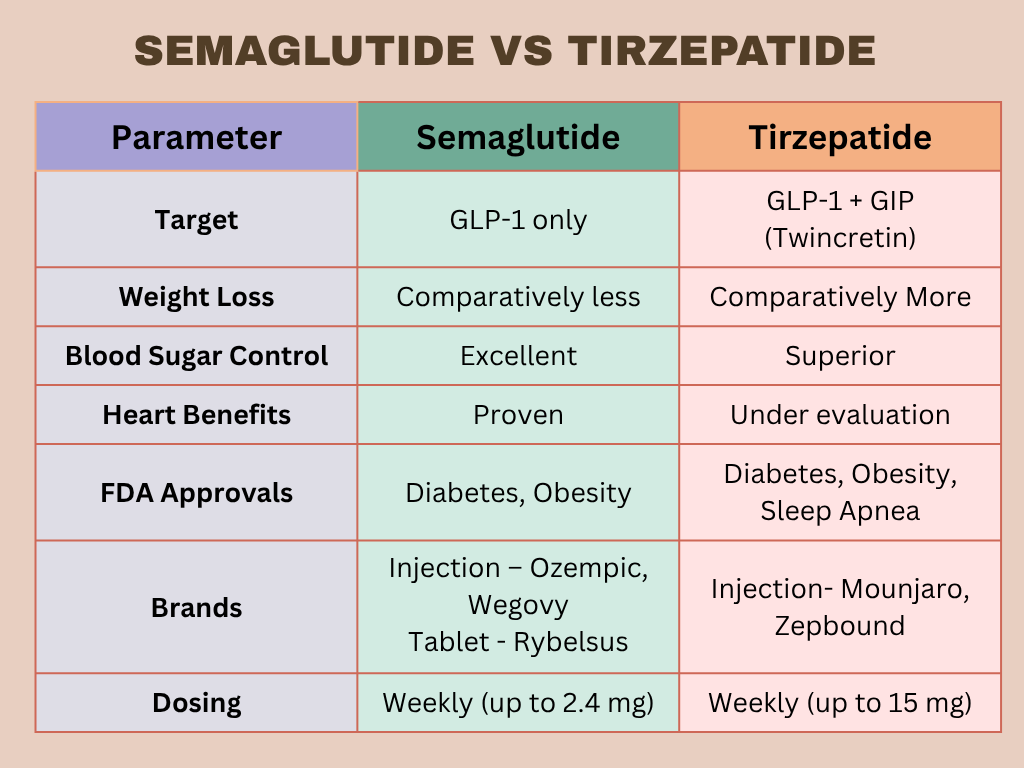
Semaglutide and Tirzepatide Have Gained Widespread Attention for Their Remarkable Effects on Weight Loss and Blood Sugar Control. Both are incretin-based treatments, but they differ in specific ways shown below (Figure 4).
🔬 Mechanism of action
- Semaglutide Mimics One of the Incretin Hormones—GLP-1—which helps regulate appetite and blood sugar. Tirzepatide, on the other hand, mimics two incretin hormones—GLP-1 and GIP. That’s why people often call it a “twincretin.” Experts believe this dual action would allow tirzepatide to reduce weight and control blood sugar more effectively.
⚖️ Weight Loss
- Tirzepatide has shown better weight loss results than semaglutide. In the SURMOUNT-5 study, people with obesity but without diabetes lost significantly more weight with Tirzepatide (Zepbound) than with Semaglutide (Wegovy). After 72 weeks, those on Tirzepatide lost an average of 20.2% of their body weight, while those on Semaglutide lost 13.7%. Nearly twice as many people lost 25% or more of their body weight with tirzepatide compared to semaglutide. Tirzepatide also led to a greater reduction in waist Circumference compared to semaglutide (Aronne et.al.,2025; Eli Lilly’s New Realease, Dec,2024).
🧪 Blood Sugar Control
- Both medications help lower blood sugar levels. In the SURPASS-2 study, tirzepatide proved more effective than semaglutide, especially at higher doses (10 mg and 15 mg). It led to greater reductions in HbA1c—a key marker of long-term blood sugar control (Frias et.l.,2021).
❤️ Cardiovascular Outcome
- studies have shown that semaglutide lowers the risk of major heart problems, like heart attack, stroke, and heart-related death (Marso et.al.,2016 Lincoff et.al.,2023).
- Early research and real-world data suggest that tirzepatide may offer even better heart protection, especially for people with type 2 diabetes (Cervantes Et.al.,2024). A major trial , (SURPASS-CVOT )whose resulted Are expected soon will Results Are Expected Soon,give clearer answers. While the early results are encouraging, more research is needed to confirm tirzepatide’s long-term heart benefits (Stephen J et.al.,2024).
💉Dosing and Administration
- Both drugs are given as weekly injections, but their doses differ:
- Semaglutide: Starts at 0.25 mg and gradually increases to a max dose of 2.4 mg (in the case of Wegovy).
- Tirzepatide: Starts at 2.5 mg and can be increased up to 15 mg weekly.
📋FDA Approvals
- Semaglutide brands Ozempic (injection) and Rybelsus (tablet) are approved for managing type 2 diabetes, while Wegovy (injection) is approved for weight loss in both diabetic and non-diabetic patients.
- Tirzepatide i brand Mounjaro (Injection) is approved for the treatment of type 2 diabetes, while Zepbound (Injection) is approved for weight loss in both diabetic and non-diabetic patients, including those with obesity-related conditions. Zepbound is also approved for managing obstructive sleep apnea in adults with obesity.
Semaglutide and Tirzepatide – Cost Comparison In India
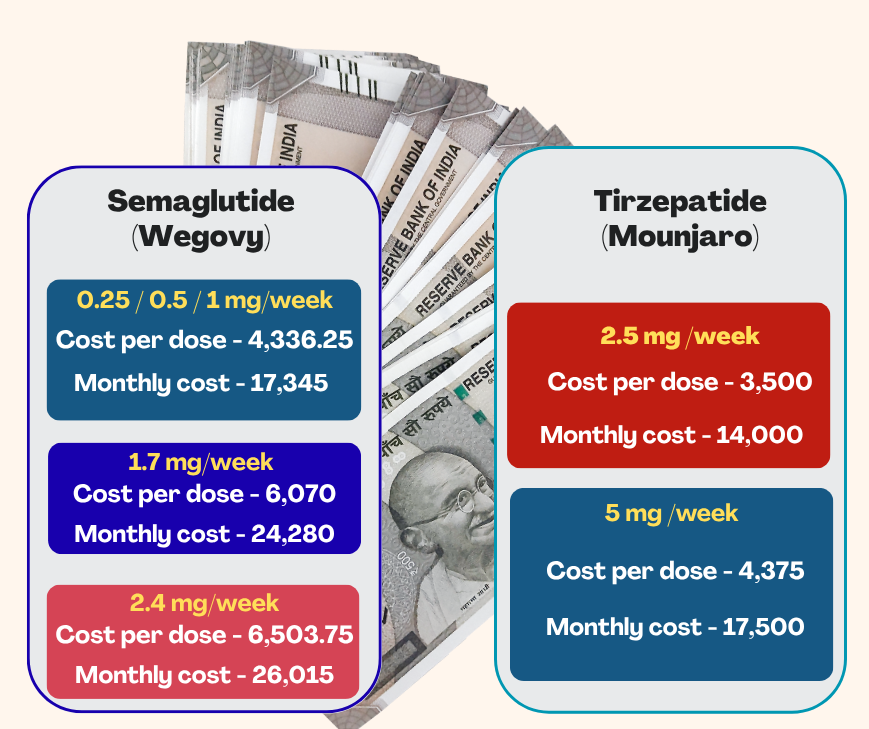
In India, the monthly cost of Semaglutide (Wegovy) ranges from ₹17,345 for the lower doses of 0.25–1 mg to ₹26,015 for the full 2.4 mg dose (FortuneIndia, June 2025). Meanwhile, Tirzepatide (Mounjaro) weekly vials are priced at ₹3,500 for 2.5 mg and ₹4,375 for 5 mg—leading to monthly costs of approximately ₹14,000–₹17,500 depending on the dose (Reuters, March 2025). As a result, tirzepatide generally offers a ₹3,000–₹6,000 monthly cost advantage over semaglutide at similar dosage levels. Both medications require a prescription and medical oversight; with semaglutide’s patent set to expire in 2026, more affordable Indian generics are expected to enter the market (Figure 5). The high expense associated with Semaglutide and Tirzepatide can create a considerable hurdle to access, especially in nations such as India, where many individuals may struggle to afford them.
Can You Switch Between Semaglutide and Tirzepatide?
Yes, you can safely switch between semaglutide and tirzepatide with your doctor’s guidance. Reasons for switching may include better blood sugar control, more weight loss, fewer side effects, lower cost, or medicine shortages. Before switching, the doctor will check your blood sugar levels, weight changes, the timing of your last dose, and how well you handled the previous medication. If both medicines are taken weekly, the new one usually starts 7 days after the last dose of the old one (Heather P et al.,2023).
Doctors usually start tirzepatide at 2.5 mg per week and increase it to 5 mg after four weeks to improve tolerability, especially in people new to GLP-1 RAs. However, in clinical studies, they directly switched patients who had been on a stable dose of semaglutide for at least three months to 5 mg of tirzepatide, with an acceptable risk of side effects(Serge et.al.,2024).
Semaglutide and Tirzepatide- Issues and Concerns
Although semaglutide and tirzepatide have demonstrated considerable effectiveness in controlling diabetes and obesity, they do come with some disadvantages. Here are a few discussed below.
Gastrointestinal side effects
- The most common Side effects reported by patients taking either Semaglutide or Tirzepatide are nausea, vomiting, diarrhoea, abdominal pain and constipation.
- These side effects are most commonly observed when starting or increasing the dose.
Risk of Pancreatitis
- Rare yet significant side effects Include Acute pancreatitis (Figure 6). It is especially common in patients with a history of pancreatic issues. So, Caution is advised in high-risk individuals.
Thyroid Cancer Risk
- Semaglutide and tirzepatide may have a possible association with thyroid cancer. Both also carry a boxed warning for the potential to cause thyroid cancer.
- A recent FDA analysis found a significant link between GLP-1 RAs like semaglutide and tirzepatide and increased reports of thyroid cancer. (Christophe et.al.,2025).
Vision Issues
- The use of semaglutide and tirzepatide has been linked to non-arteritic anterior ischemic optic neuropathy (NAION)—a rare but serious eye condition that can cause sudden, permanent vision loss (Bradley Et Al., 2025).
- A recent study indicated that patients with diabetes who are using GLP-1 receptor agonists may have over double the risk of developing neovascular age-related macular degeneration compared to those who are not on these medications. Macular degeneration is an eye disorder that affects your central vision—the area you rely on for reading, driving, and Recognizing faces (Reut et.al.,2025).
Cost and Accessibility Issues
- Semaglutide and Tirzepatide encounter considerable challenges regarding cost and accessibility in India, especially for individuals with lower socioeconomic status.
- Although these medications are currently available in India, their high prices can hinder access, potentially resulting in a black market and misuse.
Latest Updates
On August 15, 2025, the U.S. FDA granted approval to Wegovy (semaglutide) for the treatment of non-cirrhotic metabolic dysfunction-associated steatohepatitis (NASH). This marks the first-ever therapy approved for MASH, opening a new chapter in liver disease management and offering hope to millions at risk of progression to cirrhosis.
Conclusion
The launch of semaglutide and tirzepatide in India represents a Pivotal Moment in the Management of Obesity in Healthcare. These drugs bring hope to many individuals dealing with weight-related health concerns, especially when combined with lifestyle changes. However, they are not permanent solutions. Thorough medical guidance, awareness of potential adverse effects, and setting realistic expectations are essential. As enthusiasm rises, it is crucial to prioritize safe, ethical, and evidence-supported applications. Please always consult your healthcare provider to figure out whether these Treatments Are Suitable for Your Specific Situation.
Further Reading
- Kalra, S., Kapoor, N., Verma, M., Shaikh, S., Das, S., Jacob, J., & Sahay, R. (2023). Defining and diagnosing obesity in India: A call for advocacy and action. Journal of Obesity, 2023, 4178121. https://pmc.ncbi.nlm.nih.gov/articles/PMC10645500/
- Singh, A., Let, S., Tiwari, S., & Chakrabarty, M. (2023). Spatiotemporal variations and determinants of overweight/obesity among women of reproductive age in urban India during 2005–2021. BMC Public Health, 23(1), 1933. https://pubmed.ncbi.nlm.nih.gov/37798718/
- Medical Pharma Lifestyle Pulse. (2023). The new face of obesity: Revised definitions for Indians after 15 years. https://medicalpharmalifestylepulse.com/the-new-face-of-obesity-revised-definitions-for-indians-after-15-years/
- Son, J. W., & Lim, S. (2024). Glucagon-like peptide-1 based therapies: A new horizon in obesity management. Endocrinology and Metabolism, 39(2), 206–221. https://pubmed.ncbi.nlm.nih.gov/38626909/
- Jensterle, M., Kravos, N. A., Pfeifer, M., Kocjan, T., Janež, A., & Dolžan, V. (2022). Efficacy of GLP-1 RA approved for weight management in patients with or without diabetes: A narrative review. Advances in Therapy, 39(6), 2452–2467. https://pmc.ncbi.nlm.nih.gov/articles/PMC9063254/
- Wojtara, M., Flotyńska, J., Wożakowska-Kapłon, B., & Pawlik, A. (2023). Glucagon-like peptide-1 receptor agonists for chronic weight management. Advances in Medicine, 2023, 9946924. https://pmc.ncbi.nlm.nih.gov/articles/PMC10533252/
- U.S. Food and Drug Administration. (2023). FDA approves new medication for chronic weight management. https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management
- Reuters. (2024, December 20). Lilly’s weight-loss drug Zepbound wins US FDA approval for sleep apnea. https://www.reuters.com/business/healthcare-pharmaceuticals/lillys-weight-loss-drug-zepbound-wins-us-fda-approval-sleep-apnea-2024-12-20/
- GlobeNewswire. (2025, March 17). Anti-obesity drugs market: Industry trends, sales forecast, key players and global forecasts to 2035—Semaglutide, Tirzepatide lead the boom. https://www.globenewswire.com/news-release/2025/03/17/3043491/28124/en/Anti-Obesity-Drugs-Market-Industry-Trends-Sales-Forecast-Key-Players-and-Global-Forecasts-to-2035-Semaglutide-Tirzepatide-Lead-the-Global-Anti-Obesity-Drug-Market-Boom.html
- Grand View Research. (2025). GLP-1 agonists weight loss drugs market report. https://www.grandviewresearch.com/industry-analysis/GLP-1-agonists-weight-loss-drugs-market-report
- Reuters. (2025, July 7). Demand for obesity drugs shoots up in India as Lilly, Novo jostle for market share. https://www.reuters.com/business/healthcare-pharmaceuticals/demand-obesity-drugs-shoots-up-india-lilly-novo-jostle-market-share-2025-07-07/
- Shankar, A., Poojary, P., Thomas, T., Kalra, S., & Sahay, R. (2024). GLP-1 receptor agonists and delayed gastric emptying: Implications for invasive cardiac interventions and surgery. Cardiovascular Endocrinology & Metabolism, 14(1), e00321. https://pmc.ncbi.nlm.nih.gov/articles/PMC11620716/
- Moiz, A., Filion, K. B., Tsoukas, M. A., Yu, O. H., Peters, T. M., & Eisenberg, M. J. (2025). Mechanisms of GLP-1 receptor agonist-induced weight loss: A review of central and peripheral pathways in appetite and energy regulation. The American Journal of Medicine, 138(6), 934–940. https://www.sciencedirect.com/science/article/pii/S0002934325000592
- Aronne, L. J., et al. (2025). Tirzepatide as compared with semaglutide for the treatment of obesity. The New England Journal of Medicine, 393(1), 26–36. https://pubmed.ncbi.nlm.nih.gov/40353578/
- Eli Lilly and Company. (2025). Lilly’s Zepbound® (tirzepatide) superior to Wegovy® (semaglutide): Head-to-head trial results. https://investor.lilly.com/news-releases/news-release-details/lillys-zepboundr-tirzepatide-superior-wegovyr-semaglutide-head
- Frías, J. P., Davies, M. J., Rosenstock, J., Pérez Manghi, F. C., Fernández Landó, L., Bergman, B. K., Liu, B., Cui, X., & Brown, K. (2021). Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. The New England Journal of Medicine, 385(6), 503–515. https://pubmed.ncbi.nlm.nih.gov/34170647/
- Lincoff, A. M., et al. (2023). Semaglutide and cardiovascular outcomes in obesity without diabetes. The New England Journal of Medicine, 389(24), 2221–2232. https://pubmed.ncbi.nlm.nih.gov/37952131/
- Marso, S. P., et al. (2016). Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. The New England Journal of Medicine, 375(19), 1834–1844. https://pubmed.ncbi.nlm.nih.gov/27633186/
- Cervantes, M., Miles, B., & Mehta, A. (2024). Comparison of cardiovascular outcomes in patients with diabetes treated with tirzepatide versus semaglutide: A multi-institutional analysis. European Heart Journal, 45(Supplement_1), ehae666.2907. https://doi.org/10.1093/eurheartj/ehae666.2907
- Nicholls, S. J., et al. (2024). Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: SURPASS-CVOT design and baseline characteristics. American Heart Journal, 267, 1–11. https://pubmed.ncbi.nlm.nih.gov/37758044/
- Fortune India. (2024). Novo Nordisk launches blockbuster weight-loss drug Wegovy in India, prices start at ₹17,345 a month. https://www.fortuneindia.com/business-news/novo-nordisk-launches-blockbuster-weight-loss-drug-wegovy-in-india-prices-start-at-17345-a-month/
- Reuters. (2025, March 20). Eli Lilly launches weight-loss drug Mounjaro in India after drug regulator approval. https://www.reuters.com/business/healthcare-pharmaceuticals/eli-lilly-launches-weight-loss-drug-mounjaro-india-after-drug-regulator-approval-2025-03-20/
- Whitley, H. P., et al. (2023). Special report: Potential strategies for addressing GLP-1 and dual GLP-1/GIP receptor agonist shortages. Clinical Diabetes, 41(3), 467–473. https://pmc.ncbi.nlm.nih.gov/articles/PMC10338283/
- Jabbour, S., et al. (2024). Switching to tirzepatide 5 mg from glucagon-like peptide-1 receptor agonists: Clinical expectations in the first 12 weeks of treatment. Endocrine Practice, 30(8), 701–709. https://pubmed.ncbi.nlm.nih.gov/38723893/
- Wegovy. (2025). Official website. https://www.wegovy.com
- Eli Lilly. (2025). Tirzepatide official website. https://tirzepatide.lilly.com/
- Abi Zeid Daou, C., et al. (2025). Exploring connections between weight-loss medications and thyroid cancer: A look at the FDA adverse event reporting system database. Endocrinology, Diabetes & Metabolism, 8(2), e70038. https://pubmed.ncbi.nlm.nih.gov/40055991/
- Katz, B. J., et al. (2025). Ophthalmic complications associated with the antidiabetic drugs semaglutide and tirzepatide. JAMA Ophthalmology, 143(3), 215–220. https://pubmed.ncbi.nlm.nih.gov/39883468/
- Reut, A., et al. (2025). Glucagon-like peptide-1 receptor agonists and risk of neovascular age-related macular degeneration. JAMA Ophthalmology, e251455. https://pubmed.ncbi.nlm.nih.gov/40471562/
✨ AI-assisted content | Powered by ChatGPT ✨

