Introduction
Obesity and diabetes are acknowledged as significant health concerns of the 21st century, leading to higher rates of illness, death, and escalating healthcare expenses. Tirzepatide is a novel drug approved by the US Food and Drug Administration (FDA) and the European Medicines Agency to treat obesity and type 2 diabetes. Initially created for the management of type 2 diabetes, Tirzepatide’s additional advantage of aiding users in weight loss has transformed it into a worldwide sensation.
It is globally available as Zepbound for weight management and Mounjaro for diabetes and has shown encouraging results in clinical trials. However, it is crucial to consider both the potential benefits and the risks, adopting a balanced approach to its evaluation.
This blog aims to provide a concise overview of Tirzepatide in the context of treating diabetes and obesity, while also emphasizing its advantages and potential risks.
Tirzepatide – Approvals & Launches
- On May 13, 2022, the US FDA approved Tirzepatide (trade name: Mounjaro) injection as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.1
- On 8th November 2023, the US FDA approved Tirzepatide (Trade Name -ZEPBOUND) injection for chronic weight management in adults with obesity (BMI of ≥ 30 kg/ m2) or overweight (BMI of ≥ 27 kg/m2) with at least one weight-related condition (such as high blood pressure, type 2 diabetes or high cholesterol) for use, in addition to a reduced calorie diet and increased physical activity.2
- On December 20th, 2024, the U.S. FDA approved Zepbound as the first and only prescription medicine for the treatment of moderate to severe obstructive sleep apnea (in adults with obesity, to be used in combination with a reduced-calorie diet and increased physical activity .3
- Eli Lilly introduced Tirzepatide under the brand name MOUNJARO to the Indian market in March 2025.
Understanding Tirzepatide “A Twincretin”4
- Tirzepatide is a synthetic polypeptide comprising 39 amino acids.
- Tirzepatide is the first dual agonist of the incretins glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors (Figure 1).
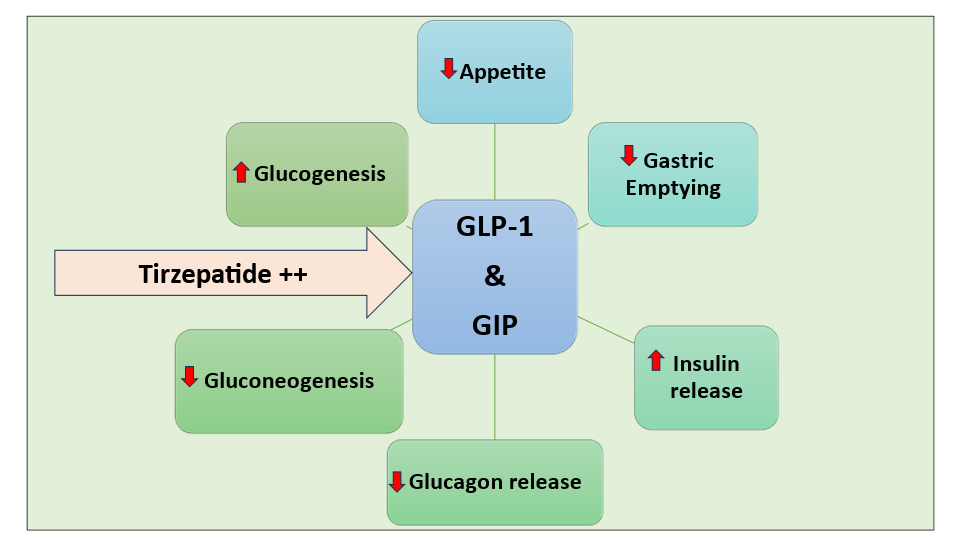
Figure 1: Mechanism of Action of Tirzepatide
- Incretins, such as GLP-1 and GIP, are hormones produced by the small intestine that regulate blood sugar levels and appetite.
- Tirzepatide enhances the activity of GLP-1 and GIP by mimicking or copying their actions.
- GLP-1 and GIP plasma concentrations rise 15 to 20 minutes after meal consumption, activating their receptors on pancreatic cells to trigger a glucose-dependent, proportionate insulinotropic response that aids in the removal of the absorbed carbohydrate and fat load.
- Since the incretin effect is diminished in diabetes, Tirzepatide is an effective alternative to achieving glycemic control.
- Due to this unique dual activity, it is also called ‘Twincretin’.
Tirzepatide – Strength Available 5-6
- Injection: 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, or 15 mg per 0.5 mL in a single-dose pen.
Tirzepatide – Dosage and Administration 5-6
- 💉 Starting dose: 2.5 mg subcutaneous (SC) injection once weekly.
- 🔄 Dose titration: After 4 weeks, increase to 5 mg SC once weekly. Further increase in 2.5 mg increments after at least 4 weeks on the current dose.
- ⬆️ Maximum dose: 15 mg SC once weekly
- ⏰ Administration time: Any time of day, with or without meals
- ⚠️ Missed dose:
- Administer promptly within 4 days (96 hours) of the missed dose
- If >4 days have passed, skip the missed dose and take the next scheduled dose
- 📍 Injection sites: Abdomen, thigh, or upper arm
- 🔁 Site rotation: Rotate injection sites with each dose
Tirzepatide- Landmark Trials in Type 2 Diabetes
SURPASS -1 Trial 7
- A 40-week, double-blind, randomised, placebo-controlled, Phase 3 trial was conducted to assess the efficacy, safety, and tolerability of once-weekly tirzepatide monotherapy in patients with type 2 diabetes who were inadequately controlled by diet and exercise alone.
- Four hundred seventy-eight participants with a mean baseline HbA1c of 7.9%, age 54, diabetes duration of 4.7 years and a BMI of 31.9 kg/m2 were enrolled in the study.
- Results
- HbA1c reductions were 1.9%, 1.9% and 2% with 5mg, 10mg and 15mg Tirzepatide, respectively.
- A dose-dependent weight loss of 7–9.5 kg was seen.
- The most frequent adverse events (AEs) with Tirzepatide were mild to moderate and transient gastrointestinal (GI) events, including nausea (12-18% vs 6%), diarrhoea (12-14% vs 8%), and vomiting (2-6% vs 2%).
- No clinically significant or severe hypoglycaemia (<54mg/dl) was reported with Tirzepatide.
SURPASS -2 Trial 8
- An open-label, 40-week, Phase 3 trial was conducted to evaluate the efficacy and safety of once-weekly tirzepatide compared to semaglutide.
- One thousand eight hundred seventy-nine people with a mean diabetes duration of 8.6 years, a mean HbA1c of 8.28%, a mean age of 56.6 years, and a mean weight of 93.7 kg were enrolled in the study.
- Results
- The mean change from baseline in the HbA1c was −2.01 %, −2.24 %, and −2.30 % with 5 mg, 10 mg, and 15 mg of Tirzepatide, respectively, and −1.86 % with Semaglutide.
- The mean reductions in body weight with Tirzepatide at a dose of 5 mg, 10 mg, and 15 mg were −7.6 kg, −9.3 kg, and −11.2 kg, respectively, as compared with −5.7 kg with Semaglutide.
- The most common AEs were GI in the Tirzepatide and Semaglutide groups (nausea, 17 to 22% and 18%; diarrhoea, 13 to 16% and 12%; and vomiting, 6 to 10% and 8%, respectively).
- Hypoglycemia (<54 mg/dL) was reported in 0.6% (5-mg group), 0.2% (10-mg group), and 1.7% (15-mg group) of those receiving 5 mg, 10 mg, and 15 mg of Tirzepatide, respectively, and in 0.4% of those who received Semaglutide.
SURPASS -3 Trial 9
- This was an open-label, 52-week phase 3 study that compared the efficacy and safety of Tirzepatide (5, 10, or 15 mg) Vs titrated Insulin degludec in 1444 type 2 diabetes patients taking metformin with or without SGLT2 inhibitors.
- The proportion of participants achieving an HbA1c < 7·0% at week 52 was greater (p<0·0001) in all three Tirzepatide groups (82%-93%) versus Insulin degludec (61%).
- Results
- At all three Tirzepatide doses, body weight decreased by between -7.5 kg and -12.9 kg, whereas in the Insulin degludec group, body weight increased by 2.3 kg.
- A higher incidence of nausea (12-24%), diarrhoea (15-17%), decreased appetite (6-12%), and vomiting (6-10%) was reported in participants treated with Tirzepatide than in those treated with Insulin degludec (2%, 4%, 1%, and 1%, respectively).
- Hypoglycaemia (<54 mg/dL) was reported in 1- 2% of participants on Tirzepatide Vs 7% on Insulin degludec.
SURPASS-4 Trial 10
- This was an open-label, parallel-group, 52-week Phase 3 study that assessed the efficacy and safety of tirzepatide versus insulin glargine in adults with type 2 diabetes and high cardiovascular risk who were inadequately controlled on oral glucose-lowering medications.
- The study enrolled a high cardiovascular-risk cohort (87% had a previous event) who had lived with diabetes for a median of 10.5 years and a mean HbA1c of 8.5% despite multiple oral antidiabetic agents.
- Results
- In a head-to-head 52-week trial vs insulin glargine U100, 5mg, 10mg and 15mg of Tirzepatide led to HbA1c reductions of 2.2%, 2.4% and 2.6%, respectively, vs 1.4% with insulin.
- Nausea (12-23%), diarrhoea (13-22%), decreased appetite (9-11%), and vomiting (5-9%) were more frequent with Tirzepatide than glargine (nausea 2%, diarrhoea 4%, decreased appetite <1%, and vomiting 2%, respectively); most cases were mild to moderate and occurred during the dose-escalation phase.
- The percentage of participants with hypoglycaemia (glucose <54 mg/dL or severe) was lower with Tirzepatide (6-9%) versus glargine.
- At 78 weeks (1166 participants) and 104 weeks (199), the Tirzepatide glycemic and weight benefits were sustained.
SURPASS – 5 Trial 11
- The study investigated the efficacy and safety of Tirzepatide added to insulin glargine in patients with type 2 diabetes who had inadequate glycemic control, with or without metformin, over 40 weeks.
- A total of 475 participants with a mean baseline HbA1c of 8.3%, age 60, diabetes duration 13.4 years, and BMI 33.4 kg/m2 received either Tirzepatide or placebo.
- Results
- Mean HbA1c reductions were 2.1%, 2.4%, and 2.3% with 5, 10, and 15 mg of Tirzepatide, respectively, compared to 0.9% in the placebo arm.
- Mean body weight reductions were 5.4kg, 7.5kg and 8.8kg with 5, 10 and 15 mg Tirzepatide compared with an increase of 1.6kg on placebo.
- Premature treatment discontinuation was high at 10–18% in the intervention arms vs 3% with placebo.
- Tirzepatide also showed statistically significant improvements in cholesterol in addition to improvements in blood pressure readings.
- The most common treatment-emergent AEs in the Tirzepatide groups vs the placebo group were diarrhoea (12%-21% vs 10%) and nausea (13%-18% vs 3%).
Tirzepatide – Clinical Trials in Type 2 Diabetes Patients with Fatty Liver Disease
SURPASS -3 MRI Trial 12
- This randomised, open-label, parallel-group study investigated the changes in liver fat content and volume of visceral and abdominal subcutaneous adipose tissue in response to Tirzepatide or insulin degludec in a subpopulation of the SURPASS-3 study.
- Five hundred two diabetic participants with a fatty liver index of at least 60 were included in the study.
- Results
- The absolute reduction in liver fat content at week 52 was significantly greater for the pooled Tirzepatide 10 mg and 15 mg groups (-8.09%) compared to the insulin degludec group (-3.38%).
- Tirzepatide showed significant improvements in visceral adipose tissue volume and abdominal subcutaneous adipose tissue.
Tirzepatide Landmark Clinical Trials in Obesity
SURMOUNT -1 Trial 13
- A 72-week phase 3 double-blind, randomised, controlled trial evaluated the efficacy and safety of Tirzepatide in non-diabetic obese patients.
- Over 2500 adults with a BMI of ≥30 kg/m2 or ≥27 kg/m2 with at least one weight-related complication were randomised to receive either once-weekly, Tirzepatide (5 mg, 10 mg, or 15 mg) or placebo.
- The mean baseline BMI was 38kg/m2, and the body weight was 104.8kg.
- Results
- The mean percentage change in weight at week 72 was between 15% and 20.9% in the Tirzepatide group, compared to 3.1% in the placebo group.
- Tirzepatide improved all predetermined cardiometabolic parameters.
- Remarkably, at 72 weeks, 95% of individuals with pre-diabetes returned to normoglycemia.
- The most common AEs with Tirzepatide were GI, and most were mild to moderate in severity.
SURMOUNT-2 Trial 14
- This Phase 3, double-blind, randomised, placebo-controlled trial investigated the efficacy and safety of Tirzepatide (10 and 15 mg) for weight management in individuals with obesity and type 2 diabetes.
- The study included over 1,500 adults with a mean body weight of 100.7 kg, BMI of 36.1 kg/m², and HbA1c of 8.02%.
- Results
- Weight reductions of 12.8–14.7% were observed with tirzepatide compared to 3.2% with placebo at 72 weeks.
- The most frequent AEs with Tirzepatide were GI, including nausea, diarrhoea, and vomiting and were primarily mild to moderate in severity.
SURMOUNT-3 Trial 15
- This double-blind, placebo-controlled trial evaluated the effect of Tirzepatide on weight reduction after a successful intensive lifestyle intervention in obese patients without diabetes.
- 579 adults with a mean BMI of 38.6 kg/m2, mean body weight of 109.5 kg and at least one obesity-related complication, who achieved ≥5.0% weight reduction after a 12-week intensive lifestyle intervention, were randomised to Tirzepatide (10 or 15 mg) or placebo once weekly for 72 weeks.
- Results
- The mean change at week 72 was −18.4% with Tirzepatide maximum tolerated dose and gain of 2.5% with the placebo.
- 87.5% of Tirzepatide-treated participants lost an additional ≥5% of their randomisation weight compared vs. 16.5% of placebo-treated participants.
- The most common AEs with Tirzepatide were GI, with most being mild to moderate in severity.
SURMOUNT- 4 Trial 16
- This Phase 3, randomised withdrawal clinical trial investigated the effect of Tirzepatide, combined with diet and physical activity, on maintaining weight reduction.
- There were 2 periods in the trial:
- 36 weeks open-label lead-in period where 783 participants received the maximum tolerated dose (10 or 15 mg) of Tirzepatide
- 52-week double-blind treatment period in which 670 participants were randomised to either continue on Tirzepatide or switch to placebo
- Results
- A 36-week lead-in period resulted in a mean weight reduction of 20.9%.
- Between 36 and 88 weeks, the mean percentage weight change was -5.5% with tirzepatide versus 14.0% with placebo.
- At 88 weeks, 89.5% of those receiving Tirzepatide maintained at least 80% of the weight loss during the lead-in period, compared to 16.6% with a placebo.
- Overall mean weight reduction from 88 weeks was 25.3% for Tirzepatide and 9.9% for placebo.
- The most common AEs were primarily mild to moderate GI events.
Tirzepatide- Promising Results from SURPASS – CVOT17
From recent reports and press releases
- Tirzepatide showed noninferiority to dulaglutide for the primary composite outcome of major cardiovascular events (MACE).
- MACE occurred 8% less frequently with Tirzepatide (HR 0.92; 95% CI 0.83-1.01), which met the pre-specified criteria for non-inferiority.
- Other outcomes:
- All-cause mortality was lower with Tirzepatide compared to Dulaglutide.
- Improvements in kidney function were reported with Tirzepatide.
- Better glycemic control (HbA1c) was observed with tirzepatide compared to dulaglutide.
- Safety and tolerability were generally consistent with their established profiles.
Tirzepatide – Side Effect Profile6
- Common Side Effects
- Nausea, diarrhea, decreased appetite, vomiting, constipation, dyspepsia, and abdominal pain
- Serious Side Effects
- Severe allergic reactions
- Thyroid tumors
- Pancreatitis (Inflammation of the pancreas)
- Hypoglycemia (Low blood sugar)
- Kidney damage
- Gall bladder diseases
- Vision changes due to diabetic retinopathy
- Severe GI diseases
Tirzepatide – Pros n Cons 18
✅ Pros
- First dual GIP + GLP-1 receptor agonist (unique mechanism)
- Robust efficacy: marked HbA1c reduction and substantial weight loss
- Superior efficacy over semaglutide (weight loss) and insulin degludec (glycemic control) in trials
- Cardiometabolic benefits: improvements in blood pressure, lipids, and fatty liver (MASH/MAFLD) outcomes
- Minimal hypoglycemia risk (when not combined with insulin or sulfonylureas)
- Convenient weekly dosing → may improve adherence
⚠️ Cons
- High cost → limits accessibility in many settings
- GI side effects are common (nausea, vomiting, diarrhoea, constipation), especially during titration.
- Cardiovascular outcomes: noninferior, but superiority not yet proven (SURPASS-CVOT)
- Debatable advantage in primary prevention populations (T2D without established CVD)
- Limited evidence in newly diagnosed T2D, lean T2D, and elderly populations
- Long-term safety and risk/benefit balance are still under investigation
Further Reading
- Eli Lilly and Company. (2022, May 13). FDA approves Lilly’s Mounjaro™ (tirzepatide) injection, the first and only GIP and GLP-1 receptor agonist for adults with type 2 diabetes. Eli Lilly and Company. https://investor.lilly.com/news-releases/news-release-details/fda-approves-lillys-mounjarotm-tirzepatide-injection-first-and
- U.S. Food and Drug Administration. (2021, June 4). FDA approves new medication for chronic weight management. https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management
- U.S. Food and Drug Administration. (2023, December 20). FDA approves first medication for obstructive sleep apnea. https://www.fda.gov/news-events/press-announcements/fda-approves-first-medication-obstructive-sleep-apnea
- Chavda, V. P, et al. (2022). Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules (Basel, Switzerland), 27(13), 4315.https://www.mdpi.com/1420-3049/27/13/4315
- StatPearls Publishing. (2023). Tirzepatide. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK585056/
- U.S. Food and Drug Administration. (2022). Mounjaro (tirzepatide) injection label (Approval Package 215866s000). https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215866s000lbl.pdf
- Rosenstock, J., et al . (2021). Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet (London, England), 398(10295), 143–155.https://pubmed.ncbi.nlm.nih.gov/34186022/
- Frias, J. P., et al . (2021). Efficacy and safety of tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. The Lancet, 398(10295), 143–155. https://pubmed.ncbi.nlm.nih.gov/34186022/
- Ludvik, B., et al. (2021). Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. The Lancet, 398(10300), 583–598. https://pubmed.ncbi.nlm.nih.gov/34370970/
- Del Prato, S., et al . (2022). Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4). The New England Journal of Medicine, 385(26), 2489–2501. https://pubmed.ncbi.nlm.nih.gov/35133415/
- Dahl, D., Onishi, et al. (2022). Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA, 327(6), 534–545.https://pubmed.ncbi.nlm.nih.gov/35133415/
- Gastaldelli, A., et al (2022). Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. The lancet. Diabetes & endocrinology, 10(6), 393–406. https://pubmed.ncbi.nlm.nih.gov/35468325/
- Jastreboff, A. M., et al. (2022). Tirzepatide once weekly for the treatment of obesity. New England Journal of Medicine, 387(3), 205–216.https://www.nejm.org/doi/full/10.1056/NEJMoa2206038
- Garvey, W. T., et al, & SURMOUNT-2 investigators (2023). Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet (London, England), 402(10402), 613–626.https://pubmed.ncbi.nlm.nih.gov/37385275/
- Wadden, T. A., Chao, et al. (2023). Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nature medicine, 29(11), 2909–2918.https://pubmed.ncbi.nlm.nih.gov/37840095/\
- Aronne, L. J., et al, & SURMOUNT-4 Investigators (2024). Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA, 331(1), 38–48.https://pubmed.ncbi.nlm.nih.gov/38078870/
- Eli Lilly and Company. (2025, July 31). Lilly’s Mounjaro (tirzepatide), a GIP/GLP-1 dual agonist, demonstrated cardiovascular protection in landmark head-to-head trial, reinforcing its benefit in patients with type 2 diabetes and heart disease. Eli Lilly and Company. https://investor.lilly.com/news-releases/news-release-details/lillys-mounjaro-tirzepatide-gipglp-1-dual-agonist-demonstrated
- Koufakis, T., Popovic, D. S., & Papanas, N. (2023). Should tirzepatide be considered for early management in type 2 diabetes? Pros and cons. Expert opinion on pharmacotherapy, 24(15), 1657–1660.https://www.tandfonline.com/doi/full/10.1080/14656566.2023.2237414#d1e184


Pingback: New Weight Loss Drugs In india- Everything you Need to Know!! -