Introduction
Obesity constitutes an epidemic and a critical public health issue. The global incidence of obesity is on the rise, showing a notable increase in obesity rates among both adults and children. With estimates indicating that over 1 billion people globally will face obesity by 2030, the growing number of overweight and obese individuals in India is a significant issue. Medical weight management stands out among the various treatment options for excess weight. Recent developments have significantly altered our treatment approaches, paving the way for future advancements in the care of obesity.
Semaglutide, marketed under the Ozempic and Wegovy brand name, has surged in popularity, driven by social media buzz and celebrity endorsements. According to reports, individuals are resorting to these medications as a “quick solution” for shedding a few kilograms; however, the potential side effects can be hazardous, necessitating their use under medical supervision. Therefore, we need to explore several important questions.
Semaglutide (Ozempic, Wegovy) : FAQs
1. What is Semaglutide?
- Semaglutide is a glucagon-like peptide 1 (GLP-1) agonist, which means it increases the activity of GLP-1.
- The small intestine produces GLP-1, a hormone that regulates blood glucose levels and modulates appetite.
2. What are the brands of Semaglutide currently available?
- The US FDA has approved Novo Nordisk Semaglutide under three distinct brand names:
- Injection – Ozempic®, Wegovy®
- Tablets – Rybelsus®
3. Are Ozempic or Wegovy currently available in India?
- In June 2025, Novo Nordisk Launched Wegovy In The Indian Market, while Ozempic is not directly available.
4. Is Rybelsus available in India?
- Novo Nordisk has marketed Rybelsus, an oral semaglutide, in India since 2022.
- Doctors prescribe it along with diet and exercise to improve blood sugar in adults with type 2 diabetes.
- It’s available in three strengths: 3mg, 7mg, and 14mg.
5. What indications has the US FDA approved for Ozempic?
- Management of type 2 diabetes along with diet and exercise.
- To reduce the risk of major cardiovascular events in adults with type 2 diabetes and established cardiovascular disease.
- To reduce the risk of sustained estimated glomerular filtration rate decline, end-stage kidney disease, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease.
6. Which are the recently approved Indications of Ozempic?
- On January 28, 2025, Novo Nordisk revealed that the U.S. FDA had approved Ozempic to reduce the risk of worsening kidney disease and cardiovascular death in adults with both type 2 diabetes and chronic kidney disease.
- The approval was based on the phase 3b results of the international FLOW kidney outcomes trial.
- Researchers enrolled over 3,500 participants with chronic kidney disease and type 2 diabetes and randomly assigned them to receive either 1 mg semaglutide weekly or a placebo, along with standard care.
- Semaglutide 1 mg demonstrated a significant 24% reduction in relative risk for the deterioration of kidney disease, the development of end-stage kidney disease, and death.
7. Has the US FDA approved Ozempic for weight loss?
- The US FDA has not approved Ozempic for aesthetic weight reduction, despite the Significant Attention It Has Garnered Through Social Media and Celebrity Influence.
8. What indications has the US FDA approved for Wegovy?
- Weight management in Obese Adults and pediatric patients ≥ 12 years.
- Doctors prescribe Wegovy alongside a reduced-calorie diet and increased physical activity for the chronic management of weight in:
- Adult patients who have an initial body mass index (BMI) of either:
- ≥30 kg/m², or
- ≥27 kg/m² when accompanied by at least one weight-related comorbidity, such as hypertension, type 2 diabetes mellitus, or dyslipidemia.
- Pediatric patients ( ≥ 12 years ) with an initial BMI at the 95th percentile or greater for age and sex.
- Adult patients who have an initial body mass index (BMI) of either:
On August 15, 2025, the US FDA approved Wegovy 2.4 mg for Noncirrhotic MASH.
👉
Read more: A New Era: FDA Approves Wegovy for Noncirrhotic MASH
9. Can Wegovy and Ozempic be considered the same?
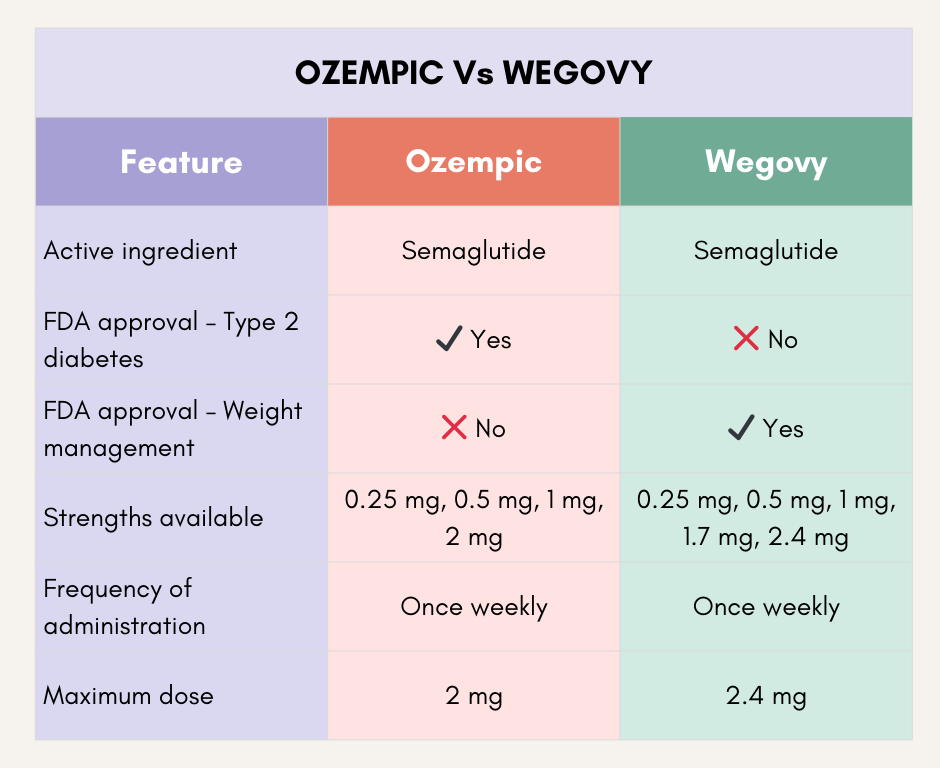
- Wegovy and Ozempic refer to the same medication, Semaglutide.
- Ozempic is specifically designated for individuals with diabetes, having received FDA approval in 2017.
- In contrast, Wegovy was approved in 2021 for those who are obese or overweight and face additional health issues.
- They are prescribed under different names, with minor variations in dosage and indications.
10. How does Semaglutide aid in weight reduction and treating type 2 diabetes?
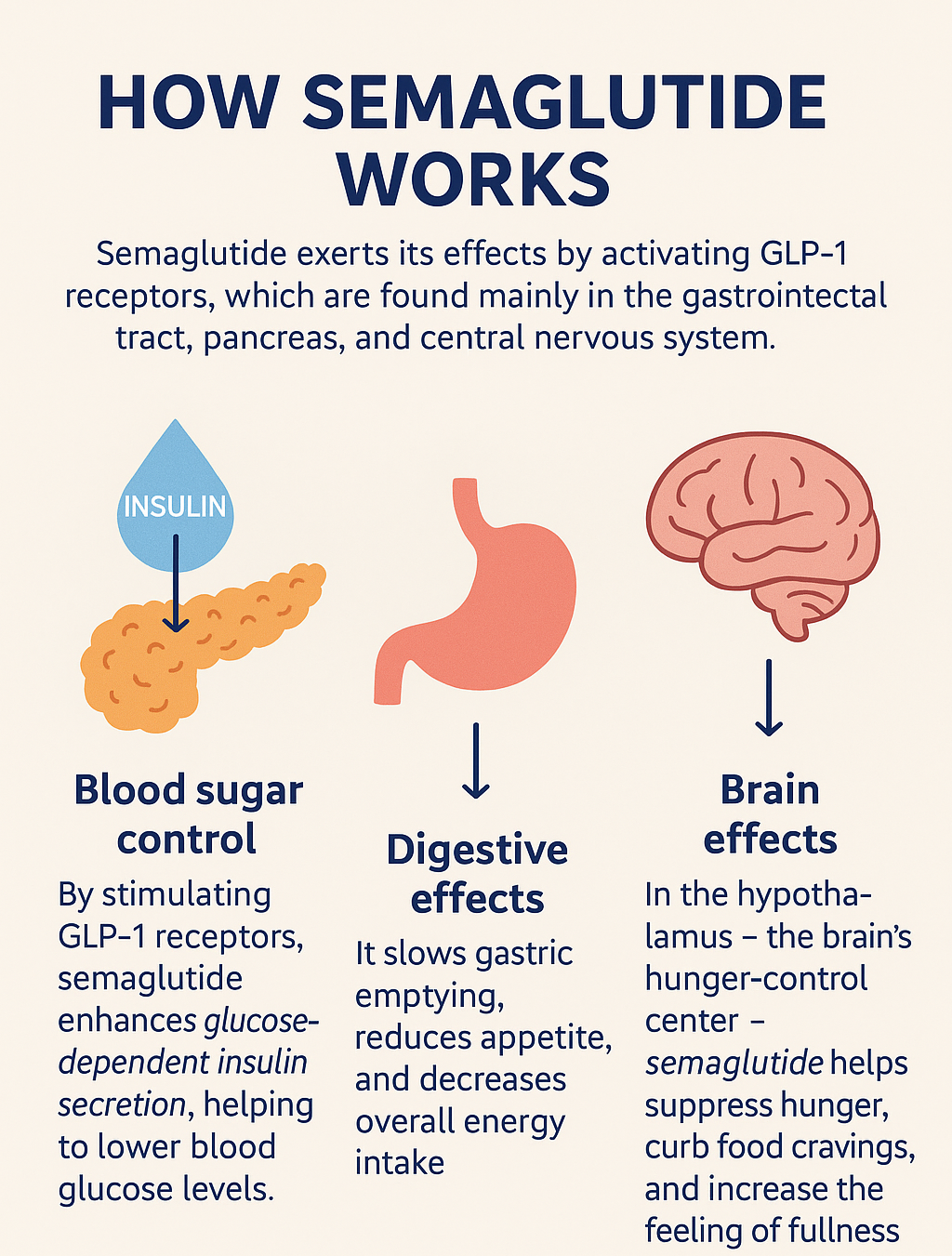
- Semaglutide delivers its benefits by activating GLP-1 receptors, primarily located in the gastrointestinal tract, pancreas, and central nervous system.
- Upon activation of the GLP-1 receptor, Semaglutide enhances glucose-dependent insulin secretion and helps to lower blood glucose levels.
- Simultaneously, it also causes delayed gastric emptying, reduced appetite and energy intake.
- In addition, Semaglutide acts on GLP-1 receptors located in the hypothalamus (a part of the brain that controls hunger) and may help suppress hunger, reduce food cravings, and increase the perception of fullness.
11. How much weight loss has been reported in clinical studies in participants taking semaglutide?
- STEP clinical trial program
- The Semaglutide Treatment Effect in People with Obesity (STEP) clinical trial program is assessing the efficacy of once-weekly subcutaneous Semaglutide at a dosage of 2.4 mg in overweight or obese individuals without diabetes.
- Throughout Steps 1, 3, 4, and 8, participants receiving Semaglutide 2.4 mg experienced mean weight losses between 14.9% and 17.4% at the 68-week.
- STEP-UP obesity trial
- On January 17, 2025, Novo Nordisk announced the results of the 72-week STEP-UP obesity trial, which included 1,407 non-diabetic adults with a BMI ≥30 kg/m².
- The results indicated that a subcutaneous dose of 7.2 mg of Semaglutide resulted in a weight loss of 20.7%, while an overall reduction of 18.7% was observed, irrespective of the participants’ adherence to the treatment.
12. How long can patients use semaglutide for weight management?
- Researchers have confirmed the long-term efficacy and safety of semaglutide for up to 2 years. It can be a viable option for managing chronic weight.
13. Does using Ozempic or Wegovy cause permanent weight loss?
- No, while Ozempic or Wegovy can produce significant and lasting weight reductions, they are not considered permanent fixes.
14. Is rebound weight gain seen with Ozempic or Wegovy?
- Yes, weight regain is common after stopping Ozempic or Wegovy.
- The medication’s impact on appetite and digestion diminishes, potentially leading to increased feelings of hunger and cravings.
- A study revealed that a year after stopping the administration of once-weekly subcutaneous Semaglutide 2.4 mg and engaging in lifestyle modifications, participants regained approximately two-thirds of the weight they had initially lost.
- Thus, maintaining a healthy lifestyle characterised by balanced eating and regular exercise can help alleviate this issue and support weight loss maintenance efforts.
15. What are the dosage forms and strengths of Ozempic and Wegovy available?
- Ozempic – Injections are available as a single-patient-use pen that delivers doses of 0.25 mg, 0.5 mg, 1 mg or 2 mg.
- Wegovy- Injections are available as a single-patient-use pen that delivers doses of 0.25 mg, 0.5 mg, 1 mg, 1.7 or 2.4 mg
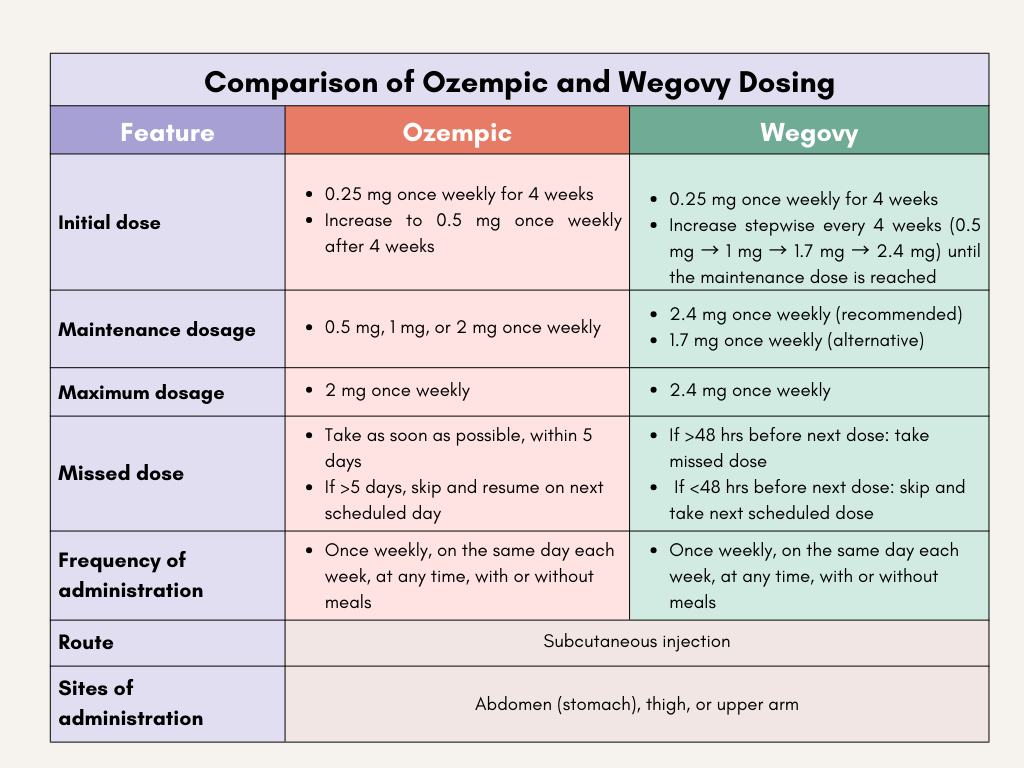
16. What is the dose, frequency and route of administration of Ozempic and Wegovy?
17. What are the common side effects of Semaglutide?
- The adverse reactions most frequently observed include nausea, vomiting, diarrhea, abdominal pain, and constipation.
18. What are the serious side effects of Semaglutide?
- Semaglutide may cause serious side effects, including:
- Possible thyroid tumours, including cancer
- Severe allergic reaction
- Pancreatitis
- Gallstones, infections of the gallbladder
- Diabetic retinopathy (damage to the eye’s retina) leading to vision issues
- Hypoglycemia (Low blood sugar)
- Acute Kidney Injury due to dehydration
- Increased heart rate
19. What is Ozempic face?
- Patients who experience rapid weight loss often report a significant loss of facial fat, leading to multiple cosmetic changes.
- These alterations can manifest as wrinkles, sunken eyes, a hollowed look, sagging jowls around the neck and jaw, and changes in the contours of the cheeks, lips, and chin. This adverse facial effect has led to the introduction of the term “Ozempic Face.”
20. What are the latest safety risks of Semaglutide, as highlighted by the new studies?
- Risk of blindness – According to a study featured in JAMA Ophthalmology, patients taking Semaglutide were found to have a greater probability of blinding eye disease, i.e. Non-arteritic anterior ischemic optic neuropathy, than those who did not take the medication. Nonetheless, additional research is essential to examine the hypothesis, and patients ought to be aware of this information until more studies are conducted.
- Gastroparesis (Stomach paralysis) – New findings have highlighted a relationship between drugs like Semaglutide and a condition termed gastroparesis. One of the prominent long-term effects of diabetes is gastroparesis, which constitutes nearly one-third of all cases. Semaglutide is chiefly employed in the treatment of type 2 diabetes mellitus. This situation highlights the importance of recognizing medication-induced gastroparesis as a potential diagnosis, particularly in those with risk factors.
- Suicidal thoughts– A new research investigation has found a correlation between the administration of Semaglutide drugs and the occurrence of suicidal ideation among patients with antidepressant use. Conversely, other recent studies have revealed that there is no connection between Semaglutide usage and depressive symptoms or suicidal tendencies in individuals who do not have existing mental health conditions.
Key Takeaways
- 💉 Ozempic and Wegovy are injectable forms of the same active ingredient, Semaglutide.
- ⚖️ Wegovy is approved for the chronic management of weight.
- 🩺 Ozempic is approved for the treatment of type 2 diabetes and for the prevention of kidney damage.
- 🌍 In India, Wegovy is currently available, while Ozempic is not officially available for sale.
- 💊 Rybelsus, the oral tablet form of Semaglutide, is available in India.
- 👩⚕️ Both Ozempic and Wegovy should be taken only under medical supervision.
- ⏳ These medications are not a permanent solution for weight loss.
- 🔄 Once discontinued, much of the lost weight can return.
- ⚠️ Misuse of Ozempic or Wegovy can increase the risk of serious complications.
- 🤢 Common side effects include nausea, vomiting, diarrhea, abdominal pain, and constipation.
Further Reading
- Alghamdi, A. A., et al. (2023). Management of obesity in low- and middle-income countries: Barriers and solutions. International Journal of Obesity, 47(12), 2243–2254. https://pmc.ncbi.nlm.nih.gov/articles/PMC10611613/
- JPRAS. (2024). Article abstract: [Details from Journal of Plastic, Reconstructive & Aesthetic Surgery]. Journal of Plastic, Reconstructive & Aesthetic Surgery. https://www.jprasurg.com/article/S1748-6815(24)00417-0/abstract
- The Lancet EClinicalMedicine. (2023). Obesity and global health outcomes: Article details. EClinicalMedicine, 58, 101926. https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(23)00059-7/fulltext
- Lee, Y., et al. (2020). Global prevalence and risk factors of obesity: Evidence review. Frontiers in Public Health, 8, 485. https://pmc.ncbi.nlm.nih.gov/articles/PMC7434819/
- Novo Nordisk. (2025). Ozempic® prescribing information. https://www.ozempic.com/prescribing-information.html
- Han, S. H et al. (2023). Public Interest in the Off-Label Use of Glucagon-like Peptide 1 Agonists (Ozempic) for Cosmetic Weight Loss: A Google Trends Analysis. Aesthetic surgery journal, 44(1), 60–67. https://pubmed.ncbi.nlm.nih.gov/37402640/
- Mahmood, T., et al. (2022). GLP-1 receptor agonists in clinical practice. Diabetes & Metabolic Syndrome, 16(1), 102–110. https://pmc.ncbi.nlm.nih.gov/articles/PMC8736331/
- Medscape. (2025). Ozempic receives new indications in chronic kidney disease. https://www.medscape.com/viewarticle/ozempic-receives-new-indications-chronic-kidney-disease-2025a10002ac
- Wilding, J. P. H., et al. (2024). [Semaglutide study]. The New England Journal of Medicine. https://www.nejm.org/doi/10.1056/NEJMoa2403347
- U.S. Food and Drug Administration. (2023). FDA approves new drug treatment for chronic weight management. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-
- Novo Nordisk. (2024). Press release. https://www.novonordisk.com/news-and-media/news-and-ir-materials/news-details.html?id=915087
- Shah, M., et al. (2022). Semaglutide safety and efficacy review. Clinical Obesity, 12(5), e12522. https://pmc.ncbi.nlm.nih.gov/articles/PMC9542252/
- Lopez, L., et al. (2022). Cardiometabolic outcomes with GLP-1 agonists. Journal of Clinical Endocrinology & Metabolism, 107(9), e3668–e3679. https://pmc.ncbi.nlm.nih.gov/articles/PMC9272494/
- Wilding, J. P. H., et al. STEP 1 Study Group (2022). Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes, obesity & metabolism, 24(8), 1553–1564. https://pubmed.ncbi.nlm.nih.gov/35441470/
- Bergmann, N. C., et al. (2023). Semaglutide for the treatment of overweight and obesity: A review. Diabetes, obesity & metabolism, 25(1), 18–35. https://pmc.ncbi.nlm.nih.gov/articles/PMC10092086/
- Klein, A., et al. (2023). Long-term outcomes of semaglutide use. Frontiers in Endocrinology, 14, 1172391. https://pmc.ncbi.nlm.nih.gov/articles/PMC10092086/
- U.S. Food and Drug Administration. (2023). Drug label: Semaglutide (Ozempic) (NDA 215256s007). https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215256s007lbl.pdf
- Novo Nordisk. (2025). Ozempic® safety and side effects. https://www.ozempic.com/how-to-take/side-effects.html
- Guglielmi G. (2025). Obesity drugs: huge study identifies new health risks. Nature, 637(8048), 1034. https://www.nature.com/articles/d41586-025-00173-5
- U.S. Food and Drug Administration. (2025). FDA approves first treatment to reduce risk of serious heart problems in adults with obesity. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or
- Hathaway, J. T., Shah, et al. 3rd (2024). Risk of Nonarteritic Anterior Ischemic Optic Neuropathy in Patients Prescribed Semaglutide. JAMA ophthalmology, 142(8), 732–739.https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2820255
- Mancini, G., et al. (2025). [GLP-1 receptor agonists safety]. Journal of Clinical Investigation, 135(2), e154535. https://pmc.ncbi.nlm.nih.gov/articles/PMC11454535/
- Park, S., et al. (2024). Safety outcomes of dual incretin agonists. Diabetes, Obesity and Metabolism, 26(4), 874–886. https://pmc.ncbi.nlm.nih.gov/articles/PMC10874596/
- Schoretsanitis, G., et al. (2024). Disproportionality analysis from World Health Organization data on semaglutide, liraglutide, and suicidality. JAMA Network Open, 7(8), e2423385. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2822453


Pingback: New Weight Loss Drugs In india- Everything you Need to Know!! -